Abstract
Introduction
As a neurological complication following haploidentical haematopoietic stem cell transplantation (haplo-HSCT), immune-mediated demyelinating diseases (IIDDs) of the central nervous system (CNS) are rare, but they seriously affect a patient's quality of life (J Neurooncol, 2012). Although several reports have demonstrated that IIDDs have a high mortality rate and a poor prognosis (J Neurooncol, 2012; Neurology 2013), a method to predict the outcome of CNS IIDDs after haplo-HSCT is not currently available. Here, we reported the largest research on CNS IIDDs post haplo-HSCT, and we developed and validated a prognostic model for predicting the outcome of CNS IIDDs after haplo-HSCT.
Methods
We retrospectively evaluated 184 consecutive CNS IIDD patients who had undergone haplo-HSCT at a single center between 2008 and 2019. The derivation cohort included 124 patients receiving haplo-HSCT from 2014 to 2019, and the validation cohort included 60 patients receiving haplo-HSCT from 2008 to 2013. The diagnosis of CNS IIDDs was based on the clinical manifestations and exclusion of other aetiologies, including infection, neurotoxicity, metabolic encephalopathy, ischaemic demyelinating disorders, and tumor infiltration. The final prognostic model selection was performed by backward stepwise logistic regression using the Akaike information criterion. The final model was internally and externally validated using the bootstrap method with 1000 repetitions. We assessed the prognostic model performance by evaluating the discrimination [area under the curve (AUC)], calibration (calibration plot), and net benefit [decision curve analysis (DCA)].
Results
In total, 184 of 4532 patients (4.1%) were diagnosed with CNS IIDDs after transplantation. Among them, 120 patients had MS, 53 patients had NMO, 7 patients had ADEM, 3 patients had Schilder's disease, and 1 patient had Marburg disease. Grades II to IV acute graft-versus-host disease (aGVHD) (p<0.001) and chronic GVHD (cGVHD) (p<0.001) were identified as risk factors for developing IIDDs after haplo-HSCT. We also tested immune reconstitution by measuring the following parameters 30, 60, and 90 days after haplo-HSCT: proportions of CD19+ B cells, CD3+ T cells and CD4+ T cells; counts of lymphocytes and monocytes; and levels of immunoglobulins A, G, and M. These parameters showed no significant differences between patients with and without IIDD.
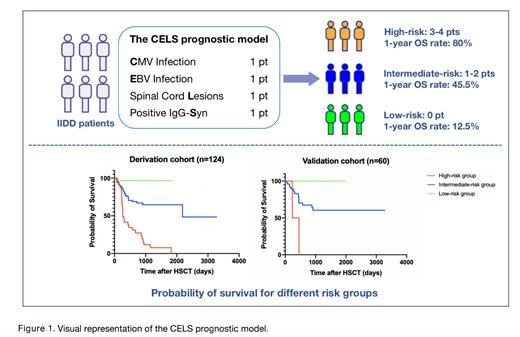
CNS IIDDs were significantly associated with higher mortality and a poor prognosis (p<0.001). In a/the multivariate logistic analysis of the derivation cohort, four candidate predictors were entered into the final prognostic model: cytomegalovirus (CMV) infection, Epstein-Barr virus (EBV) infection, the cerebrospinal fluid (CSF) IgG synthesis index (IgG-Syn), and spinal cord lesions. The value assignment was completed according to the regression coefficient of each identified independent prognostic factor for CNS IIDDs in the derivation cohort to establish the CELS risk score model. According to the regression coefficient, point values were given to each factor based on the log scale, and 1 point was awarded for each variable. These 4 factors determined the total risk score, ranging from 0 to 4. There was a higher risk of death in IIDD patients with higher CELS scores and we, therefore, defined three levels of risk of death in IIDD patients: a low-risk group for patients with a score of 0, a medium-risk group for patients with a total score of 1 or 2, and a high-risk group for patients with a total score of 3 or 4. The prognostic model had an area under the curve of 0.864 (95% CI: 0.803-0.925) in the internal validation cohort and 0.871 (95% CI: 0.806-0.931) in the external validation cohort. The calibration plots showed a high agreement between the predicted and observed outcomes. Decision curve analysis indicated that IIDD patients could benefit from the clinical application of the prognostic model.
Conclusions
We identified the risk factors for IIDD onset after haplo-HSCT, and we also developed and validated a reliable prediction model, namely, the CELS, to accurately assess the outcome of IIDD patients after haplo-HSCT. Identifying IIDD patients who are at a high risk of death can help physicians treat them in advance, which will improve patient survival and prognosis.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal